A Building Suffering From Galvanic Action
Architecture is not just the artistic composition of forms for aesthetic purposes but also the assembly of tens of thousands of individual components for specific practical purposes. Without understanding the complicated interaction between all these different parts, the pieces will begin to fail far earlier than they should. One such reaction that most owners and builders are not aware of is Galvanic Action, which is a form of electrochemical corrosion. This process poses a significant threat to the longevity and structural integrity of buildings. The phenomenon occurs when two dissimilar metals come into contact with an electrolyte (like water or moisture).
Essentially in a battery electrons flow from one metal to the other, causing metals to deteriorate rapidly and prematurely.

Electrochemical Corrosion
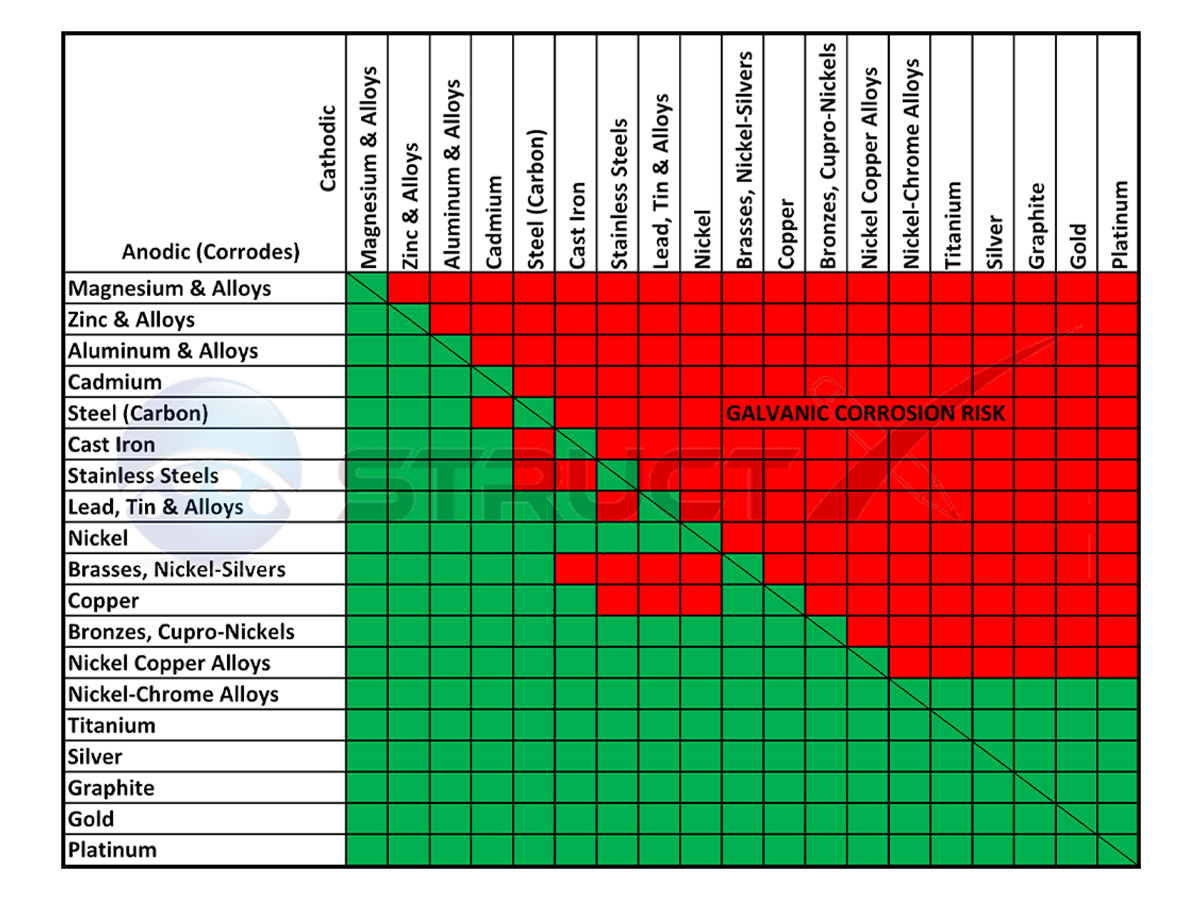
Electrochemical Disparity: Metals possess varying tendencies to lose electrons. In a “galvanic couple”, the more “active” metal (higher on the galvanic series) acts as the anode, readily releasing electrons. The less active metal (cathode) accepts these electrons.
Current Flow: This electron transfer creates an electrical current that flows between the metals through the electrolyte.
Accelerated Corrosion: The anode experiences accelerated corrosion as it loses metal ions into the electrolyte. The cathode, on the other hand, remains largely unaffected.
Why is it important to understand and avoid Galvanic action? The consequences of unchecked galvanic action can be severe. These problems can include:
Structural Weakness: Deterioration of critical metal components, such as structural steel, can compromise the building’s stability and safety.
Leaks and Infiltration: Corrosion of roofing materials, flashing, or gutters can lead to leaks, allowing water infiltration that can damage interior finishes and cause further deterioration.
Aesthetic Damage: Visible corrosion can significantly diminish the building’s appearance, impacting its value and curb appeal.
Additional Maintenance Costs: Repairing or replacing corroded components is expensive and disruptive, leading to increased maintenance costs over the building’s lifespan.

Galvanic Action In Steel
What strategies can be used to avoid this highly negative effect?
Metal Selection: The most important approach is to choose compatible metals that are close together on the galvanic series. For example, avoid coupling steel with copper or aluminum.
Protective Coatings: Apply high-quality coatings to prevent moisture from reaching the metal surfaces.
Insulation: It is also possible to physically isolate dissimilar metals using non-conductive materials like plastic or rubber.
Cathodic Protection: Lastly, in some instances, it may make sense to employ sacrificial anodes (made of a more active metal) to divert the corrosive current away from the primary structure. These sacrificial materials will need to be replaced on a specific schedule.
Regular Inspection and Maintenance: Conduct periodic inspections to identify and address any signs of corrosion early on.
By understanding the principles of galvanic action and implementing appropriate mitigation strategies, architects and engineers can significantly enhance the durability and longevity of their structures, ensuring their safety and aesthetic appeal for years to come. This is just one of hundreds of reasons why building owners need to hire capable design professionals and fully utilize their knowledge and experience throughout the design and construction process. If you have a design challenge in New England (and Rhode Island in particular) we hope you will reach out to A4 Architecture to assist you in creating a great and long-lasting) building!
Ross Sinclair Cann, AIA, LEED AP, is an historian, author, educator and founding Principal of A4 Architecture Inc. He majored in Molecular Biochemistry and Biophysics in college and holds architectural and history degrees from Yale, Cambridge and Columbia Universities.
